
COVID-19 pill

An experimental treatment from Shionogi & Co Ltd (4507.T) has shown rapid clearance of the virus that causes COVID-19, according to new data, the Japanese drug maker said on Sunday. The pill, S-217622, "demonstrated rapid cleara..
.jpg)
India gave emergency use authorisation (EUA) for Merck's (MRK.N) COVID-19 pill molnupiravir, and Serum Institute of India's Covovax and Biological E's Corbevax coronavirus vaccines, the country's health minister said on Twitter. So..
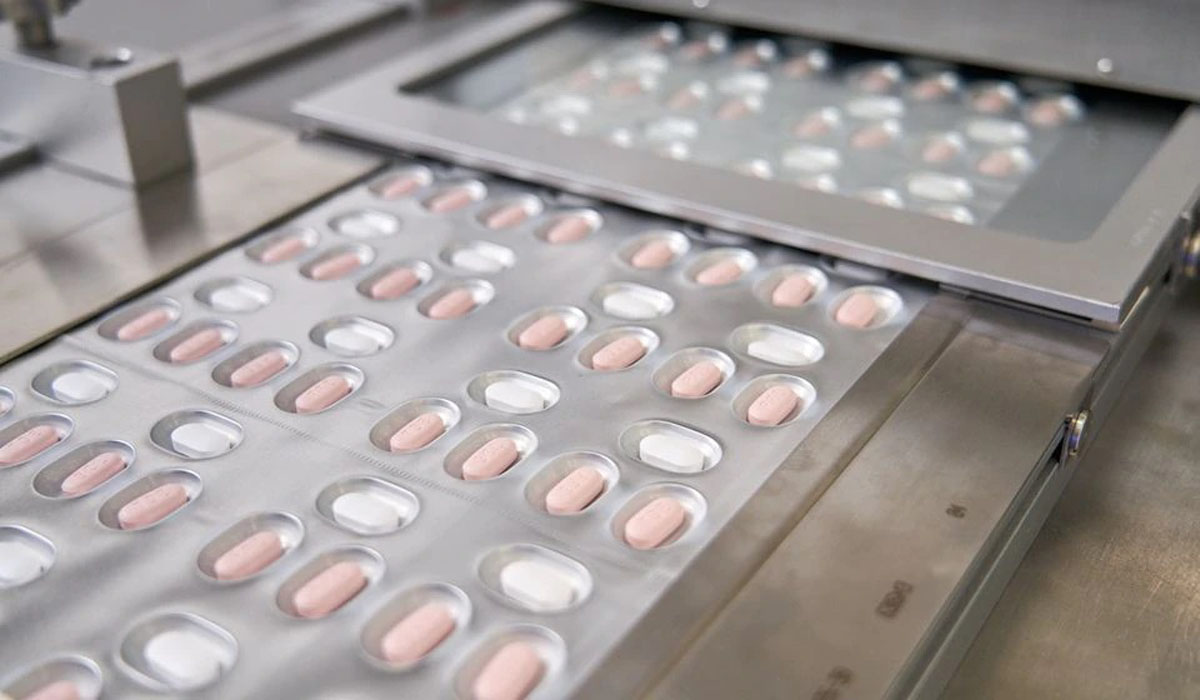
Pfizer Inc (PFE.N) said on Tuesday it will allow generic manufacturers to supply its experimental antiviral COVID-19 pill to 95 low- and middle-income countries through a licensing agreement with international public health group Medicines ..

Oral antiviral pills from Merck & Co (MRK.N) and Pfizer Inc (PFE.N) have been shown to significantly blunt the worst outcomes of COVID-19 if taken early enough, but doctors warn vaccine hesitant people not to confuse the benef..

Pfizer Inc (PFE.N) is in discussions with 90 countries over supply contracts for its experimental COVID-19 pill, which was shown to reduce by 89% the risk of hospitalization or death in patients at high risk of severe illness, Chief Executi..

Britain on Thursday became the first country in the world to approve a potentially game-changing COVID-19 antiviral pill jointly developed by U.S.-based Merck & Co Inc (MRK.N) and Ridgeback Biotherapeutics, in a boost to the fight again..
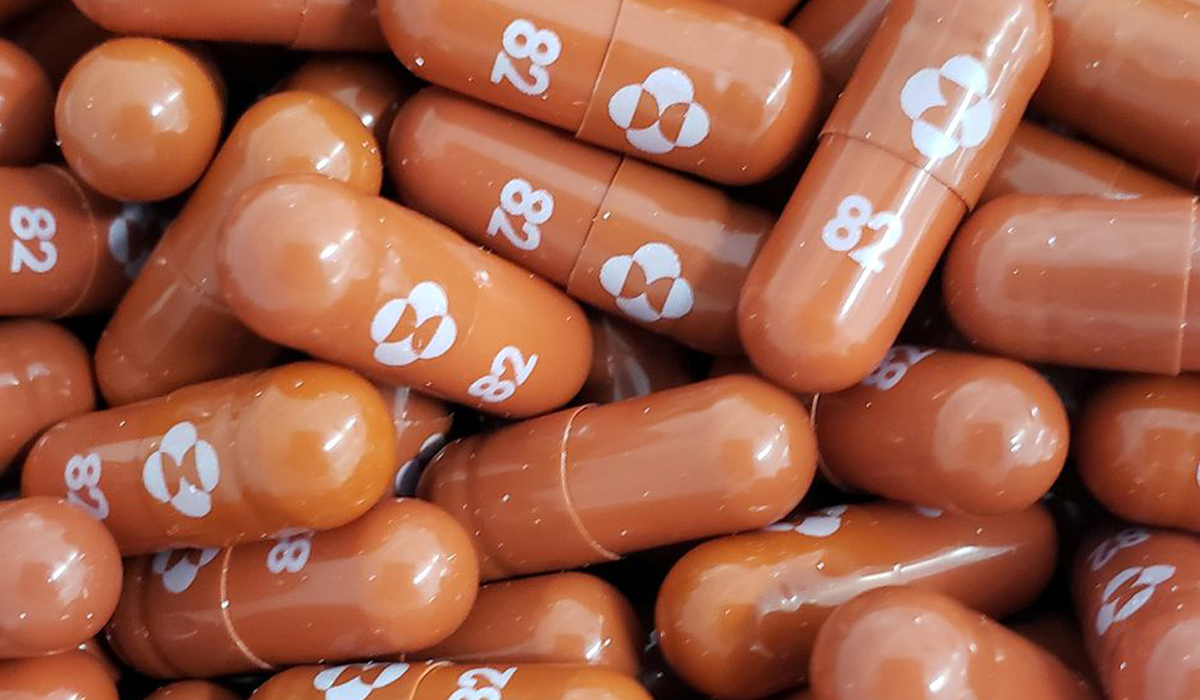
An antiviral pill developed by U.S. drugmaker Merck & Co (MRK.N) could half the chances of dying or being hospitalized for those most at risk of contracting severe COVID-19, with experts hailing it as a potential breakthrough in how the..
.jpg)
Qatar Secures Place Among the World's Top 10 Wealthiest Nations
.jpg)
Hamad International Airport Witnesses Record Increase in Passenger Traffic

Saudi Arabia: Any visa holder can now perform Umrah

What are Qatar's Labour Laws on Annual Leave?